Over the years, I ended up with some simple rules for what I will buy. The rules are simple:
- Trademarked/copyrighted/patented species, ideally ones with some research. All of them are listed on this page with links to the research. Ideally, just the one researched for your conditon.
- Single species with (almost) no fillers. There are precisely three sources that I use:
- Custom Probiotics :they list all of their strains — many are researched on the above list. No other ingredients just the bacteria.
- Maple Life Science™: No strains yet, but shipments usually have manufactured date within 4 weeks of arrival (i.e. FRESH). Contains FOS. EBAY BASED — Ships world wide, best pricing!
- Bulk Probiotics: US based Newbie — but has some species not available at the other two sites. No other ingredients just the bacteria. Specifically, Lactobacillus Jensenii that has great potential for Crohn’s disease.
- NOTE: none of these sell though retail outlets. This keeps their costs down and their product fresh.
Personal Experience
This family experience with all of the single strain probiotics has been:
- If we have not used before, we notice changes within 1-3 days in stools, symptoms, etc. These may not always be desired symptoms (i.e. one probiotic before bed, kept us awake all night; other probiotics give us deeper and longer sleep than normal). In some cases, changes are observed within 60 minutes. Changes of stools are a common change.
- We usually do one new probiotic at a time for 2 weeks to get “a feel” for what it does
- We constantly rotate – never more than 1 month on any probiotic “It has done what it is going to do“
“If there is no changes, the probiotic is dead, Jim” or not of benefit to you. Move on. A common observation that we saw using most probiotics purchased from local stores.
Medical Claims
I do not trust any claims on the bottle
The outcome of the first series of health claim applications for probiotics in Europe as evaluated by the European Food Safety Authority (EFSA) has, up to 2013 almost completely yielded negative results. All recent applications also have been rejected, including the latest on prevention of mastitis in breastfeeding mothers.
The ascent of the blessed: regulatory issues on health effects and health claims for probiotics in Europe and the rest of the world [2018]
I don’t trust retail mixtures with good reasons:
- Assessment of commercial probiotic bacterial contents and label accuracy [2011]
- Misspelled organisms: 32% — using outdated names higher!
- I.e. it is Limosilactobacillus reuteri and not Lactobacillus Reuteri
- it is Lacticaseibacillus casei and not Lactobacillus casei
- Just 27% of claims of viable organisms met or exceeded their label claim
- Misspelled organisms: 32% — using outdated names higher!
- Evaluation of deficiencies in labeling of commercial probiotics [2003]
- Misspelled organisms: 25%
- Bacterial species were misidentified 35-43% of the time
- Misidentifications included stating a name that had been changed
- i.e. “Streptococcus faecium” and not Enterococcus faecium
- Non existent species: “Lactospore sporogenes“
- Identification and antibiotic resistance of isolates from probiotic products. Abstracts of 101st ASM General Meeting. The American Society for Microbiology, Washington DC, USA,
- for 30 dried probiotic supplements tested,
- 11 contained no viable bacteria,
- only 7 contained all claimed species
- 18 had species other than those on-label.
- Deficiencies in microbiological quality and labelling of probiotic supplements [2002]
- 63% of the UK products tested were below standard.
- Enterococcus faecium (not stated on label) and was labelled with the misleadingand invalid name ‘‘L. bifidus’’.
- Microbiological Quality and Antimicrobial Resistance of Commercial Probiotic Products for Food-Producing Animals [2024]
- 64.4% were incorrectly labeled in either number of viable cells or bacterial species
- 51.6% exhibited resistance to at least one antimicrobial agent
- 26.8% had a lower number of viable cells than their label claims, No viable Lactobacillus was found in some products
- 57.8% comprised other species rather than those claimed on the contents
- More Information Needed on Probiotic Supplement Product Labels [2019]
- This cites this as the minimum recommended standard.
- Genus and species names, which should adhere to current scientifically valid nomenclature.
- Strain designations for each strain in the product. Designations used should enable tracking of the strain to entries in strain depositories and linking to published studies.
- Statement of quantity (using CFU or other validated measure) of live/active microorganisms through the use-by date.
- Use-by date.
- Statement of benefit is not required, but if present must be supported by a human study showing the benefit at the dose delivered in the product.
- Proper storage conditions Required.
- Company contact information.
- Warns of genetic drift (bacteria mutations) and cites the popular
- L. rhamnosus (GG) that exhibited multiple genotypes in consumer products (Sybesma et al., 2013). So the bottom of LGG you buy may not be the same strain as the studies used.
- This cites this as the minimum recommended standard.
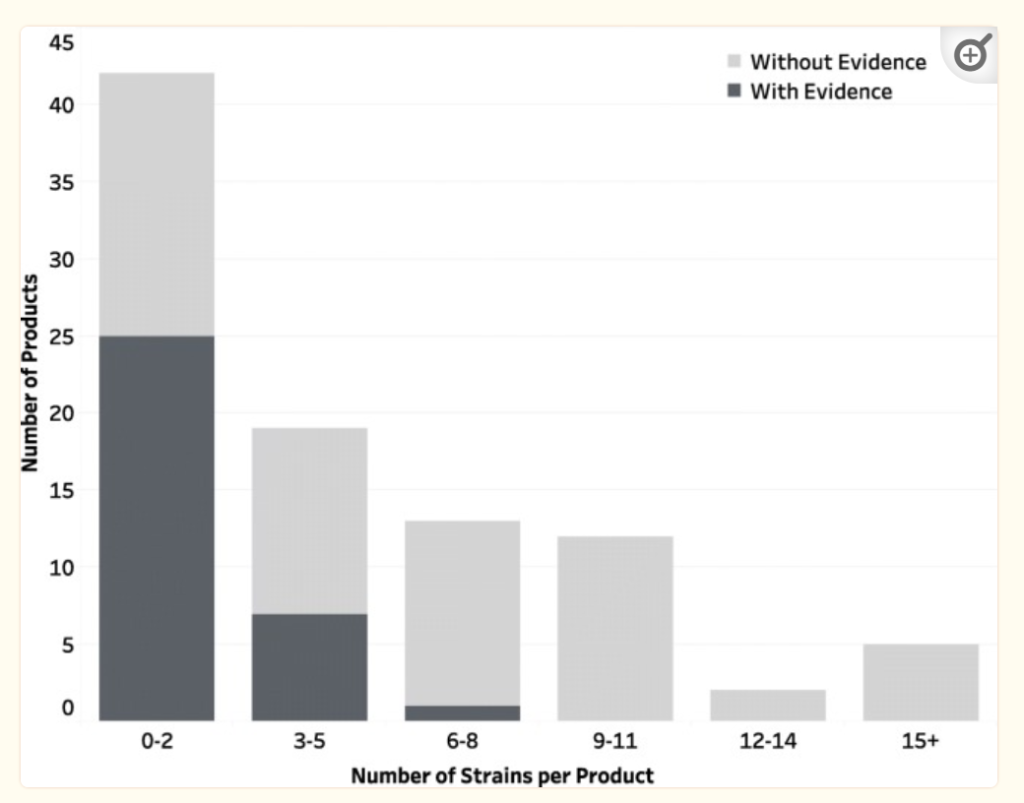
- Improving End-User Trust in the Quality of Commercial Probiotic Products [2019] shows the evidence issue, manufacturer will toss the kitchen sink into a blend and make questionable claims to promote sales.
Recent Comments