Back Story
My history is:
- currently 56 years old
- CFS started at age 32 after a bad cold
- I’ve had bloating and burping since hospitalized as a child for a lump on my throat. I received antibiotics at this time
- I went into 80% remission for about 6 years from age 50-56. I don’t know what did it but I was on a low FODMAPS diet and started using hydrogen peroxide as a mouth rinse
- Symptoms
- in addition to the bloating and burping I have the following symptoms
- fatigue
- sometimes a numb feeling in parts of my hands and feet
- orthostatic intolerance (although I did the tilt table test and tested negative)
- halitosis
- tinnitus
- a strange feeling in my head
- shortness of breath
I took inulin before my remission and my symptoms intensified immensely (especially burping and fatigue and shortness of breath)
I had a culture of my upper duodenum done in 2013 and it showed 10000CFU/ml of rothia, prevotella melaninogenica and streptococci viridins. A recent Bristle Health oral biome test showed the prevotella melaninogenica in the 90th percentile
Analysis
I was not surprised about getting ME/CFS after a cold. Cold virus include COVID which can cause Long COVID — a sibling of ME/CFS. The wrong cold virus combined with other catalysts can send someone down that path.
First, I look at the distribution of percentiles. A normal/typical microbiome should have the same count (percentage) in each of the 10%ile. As is often seen with ME/CFS and Long COVID, we have a major overrepresentation of the 0-9%ile — 4x the count of most other groups.
| Percentile | Genus | Species |
|---|---|---|
| 0 – 9 | 62 | 89 |
| 10 – 19 | 14 | 22 |
| 20 – 29 | 15 | 22 |
| 30 – 39 | 16 | 16 |
| 40 – 49 | 15 | 19 |
| 50 – 59 | 20 | 25 |
| 60 – 69 | 10 | 14 |
| 70 – 79 | 18 | 22 |
| 80 – 89 | 20 | 17 |
| 90 – 99 | 21 | 35 |
I interpret this as a host, “a mafia”, of odd bacteria that cross support each other and pumps disrupting metabolites (chemicals) into the body. Thus it is not a bacteria(person) that causes the problems but a big gang of bacteria.
There was not a strong bacteria that predominate this shift, Phocaeicola massiliensis was the only candidate. Looking at Potential Medical Conditions Detected, there were no significant matches (not surprising with an abundance of low percentile bacteria).
Bacteria deemed unhealthy is a pretty long list.
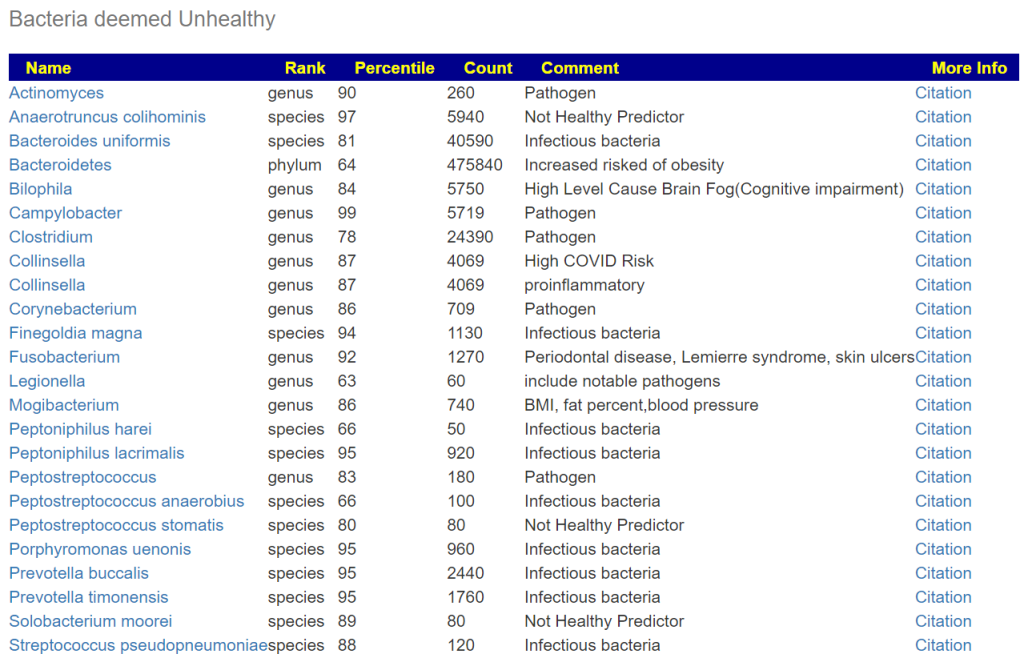
Looking at Dr. Jason Hawrelak Recommendations, we see several that are usually low percentiles that are too too low. These include: Bifidobacterium, Lactobacillus, Methanobrevibacter, Roseburia and Faecalibacterium prausnitzii.
| Taxonomy | Rank | Low | High | Your Value | Status |
|---|---|---|---|---|---|
| Bacteroidia | class | 0 | 35 | 37.564 | Not Ideal |
| Akkermansia | genus | 1 | 3 | 3.225 | Not Ideal |
| Bacteroides | genus | 0 | 20 | 29.183 | Not Ideal |
| Bifidobacterium | genus | 2.5 | 5 | 0.015 | Not Ideal |
| Blautia | genus | 5 | 10 | 6.53 | Ideal |
| Desulfovibrio | genus | 0 | 0.25 | 0.149 | Ideal |
| Eubacterium | genus | 0 | 15 | 0.006 | Ideal |
| Lactobacillus | genus | 0.01 | 1 | 0.002 | Not Ideal |
| Methanobrevibacter | genus | 0.0001 | 0.02 | 0 | Not Ideal |
| Roseburia | genus | 5 | 10 | 1.154 | Not Ideal |
| Ruminococcus | genus | 0 | 15 | 3.9 | Ideal |
| Proteobacteria | phylum | 0 | 4 | 3.09 | Ideal |
| Bilophila wadsworthia | species | 0 | 0.25 | 0.575 | Not Ideal |
| Escherichia coli | species | 0 | 0.01 | 0.006 | Ideal |
| Faecalibacterium prausnitzii | species | 10 | 15 | 5.159 | Not Ideal |
Looking at some of the conditions we see a marginally better match for Long COVID than for ME/CFS! Not sufficient to ascribe to a cold virus as the onset cause, but interesting.
Going over to our special studies, we see Long COVID is the best match

Bristle Health Results
This is the first time that I have seen this report. It is a mouth test from BristleHealth.com (good name, better then ToothBrushHealth!). I have pointed out the importance of the mouth in prior posts: A mouth full – for better or worst [2014] and Your mouth can trigger flares[2017].
It provides information on selected strains by role:

As an exercise to understand the “end-to-end” process (literally), I have created the table below. Since the data is by species we have an issue of different tests reporting different species. For more details see The taxonomy nightmare before Christmas… The * indicate that there was no match at the strain level, so we use the genus as a proxy.
First thing to remember is that bacteria is pH sensitive so the quantity in each location is expected to be very different.
| Bacteria | Bristle | Percentile | |
| Actinomyces dentalis | 1.8 | 90 | * |
| Actinomyces graevenitzii | 1.9 | 90 | * |
| Actinomyces odontolyticus | 0.4 | 90 | * |
| Atopobium parvulum | 6.8 | 69 | * |
| Atopobium sp | 8 | 69 | * |
| Campylobacter concisus | 5.2 | 98 | * |
| Campylobacter gracilis | 5.1 | 98 | * |
| Candida albicans | 1.8 | ||
| Candida dubliniensis | 1.5 | ||
| Candida glabrata | 2.2 | ||
| Candida sp | 1.1 | ||
| Capnocytophaga granulosa | 7.3 | ||
| Capnocytophaga sputigena | 0.19 | * | |
| Capnocytophaga sp | 0.18 | * | |
| Corynebacterium matruchotii | 8.3 | 86 | * |
| Dialister micraerophilus | 0.4 | 94 | * |
| Eikenella corrodens | 2.6 | ||
| Enterobacter cloacae | 2.5 | ||
| Fusobacterium nucleatum | 1.9 | 19 | |
| Fusobacterium sp | 0.3 | 92 | |
| Gemella haemolysans | 1.8 | 48 | * |
| Gemella morbillorum | 0.12 | 48 | * |
| Granulicatella adiacens (30%) | 4.8 | 15 | |
| Haemophilus haemolyticus | 4.8 | ||
| Haemophilus parainfluenzae | 7.2 | ||
| Haemophilus pittmaniae | 0.79 | ||
| Helicobacter pylori | 2.3 | 61 | * |
| Lactobacillus fermentum | 3.5 | 5 | * |
| Leptotrichia trevisanii | 0.99 | ||
| Megasphera micronuciformis | 8.5 | 24 | * |
| Neisseria elongata | 0.15 | * | |
| Neisseria flavescens | 2.1 | ||
| Neisseria mucosa | 3 | ||
| Neisseria subflava | 1 | ||
| Oribacterium sp | 0.098 | 26 | |
| Porphyromonas sp | 2 | 100 | |
| Porphyromonas catoniae | 0.27 | 100 | * |
| Prevotella sp | 10 | 73 | |
| Prevotella fusca | 2.2 | 73 | * |
| Prevotella histolica | 9.5 | 73 | * |
| Prevotella loescheii | 1.6 | 1 | |
| Prevotella melaninogenica | 9 | 73 | * |
| Prevotella pallens | 0.1 | 73 | * |
| Prevotella salivae | 8.8 | 73 | * |
| Prevotella tannerae | 0.56 | 73 | * |
| Prevotella veroalis | 7.5 | 73 | * |
| Propionibacterium acidifaciens | 2.1 | ||
| Rothia aeria | 0.8 | ||
| Rothia mucilaginosa | 5.2 | ||
| Selenomonas noxia | 4.4 | 72 | * |
| Solobacterium moorei | 0.3 | 89 | |
| Stomatobaculum longum | 0.18 | ||
| Streptococcus constellatus | 2.7 | 48 | * |
| Streptococcus infantis | 7.9 | 48 | * |
| Streptococcus intermedius | 1.9 | 0 | |
| Streptococcus mitis | 7.8 | 48 | * |
| Streptococcus oralis | 7.2 | 1 | |
| Streptococcus parasanguinis | 1.3 | 49 | |
| Streptococcus peroris | 1.6 | 48 | * |
| Streptococcus salivarius | 5 | 48 | * |
| Streptococcus sanguinis | 2 | 48 | * |
| Streptococcus tigurinus | 8.7 | 48 | * |
| Tannerella sp | 0.88 | ||
| Treponema sp | 0.73 | ||
| Veillonella atypica | 8.5 | 18 | |
| Veillonella dispar | 7.5 | 18 | * |
| Veillonella sp | 2.7 | 18 | * |
I noticed something interesting with strains that were in both samples.
- Solobacterium moorei in both. It occurs only is 14% of Biomesight samples.
- Streptococcus parasanguinis in both. It occurs only is 59% of Biomesight samples.
- Streptococcus oralis in both. It occurs only is 53% of Biomesight samples.
- Streptococcus intermedius in both. It occurs only is 13% of Biomesight samples.
- Prevotella loescheii in both. It occurs only is 15% of Biomesight samples.
- Granulicatella adiacens in both. It occurs only is 30% of Biomesight samples.
- Fusobacterium nucleatum in both. It occurs only is 11% of Biomesight samples.
My subjective conclusion is that this strongly supports the hypothesis that the mouth microbiome feeds the microbiome of the rest of the digestive track. We have 4 rare strains in the above list of 7 where only 1 would be expected to be below 14% (1 in 7).
We can get suggestions for the mouth approximately by using this feature and entering by the genus items above 7 for high, 9 for very high or below 3 for low, below 1 for very low.
| All Bacteria [Genus] Reported | 720 Bacteria |
Suggestions include (for mouth) are below. This is an experiment to see how suggestions for the “other end” compares! Values below 0.4 are usually low significance.
There are some subjective issues in entering the contents and getting suggestions. For suggestions, doing parent and/or children can be debated many ways. Some species are low of a genus and others are high… do you mark the genus high, low or normal? It is unclear if the bacteria listed are pure bad or just bad in excess. That is, should anything not zero be deemed too high or only those over 7. Things are not sufficiently cleared from this report. If I get more requests to do analysis of Bristol Health reports, I will invest more time and add a custom manual entry page. I will need to research every single species to know the appropriate handling.
Below is for purposes of illustration only
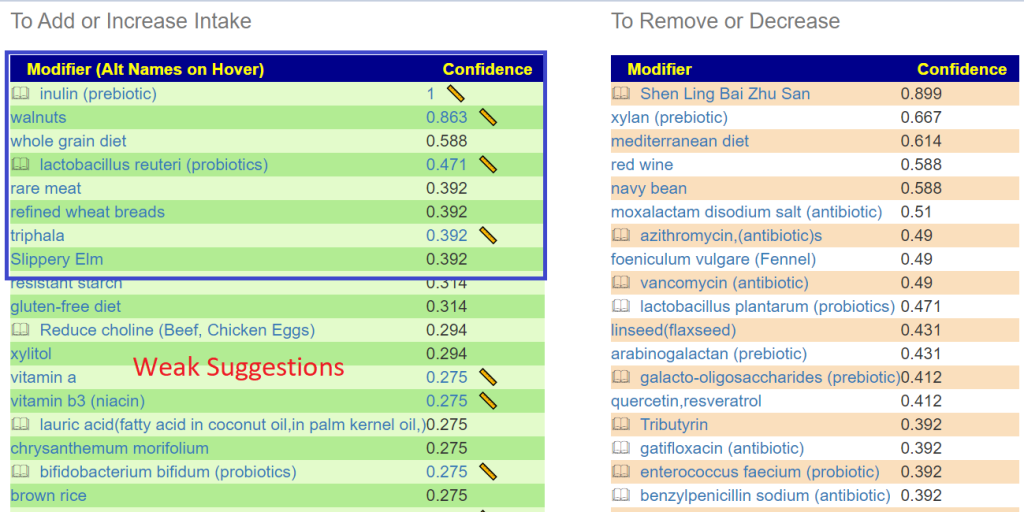
Avoids include: xylan (prebiotic), lactobacillus plantarum (probiotics), berberine, Cacao, d-ribose
Microbiome Suggestions
The adage “No man can server two master” is good to keep in mind in this scenario.
I did the usual “Just Give Me Suggestions” path since there was nothing that stood out that could require special handling. “Just Give Me Suggestions” obtains 4 sets of suggestions using different logic to try to derive the best suggestions. I will start by taking the above list and see how they rank in terms of the microbiome.
| Modifier (Alt Names on Hover) | Mouth Confidence | BiomeSight Confidence |
|---|---|---|
| 🕮 inulin (prebiotic) | 1 📏 | -27 |
| walnuts | 0.863 📏 | 6 |
| whole grain diet | 0.588 | -14 |
| 🕮 lactobacillus reuteri (probiotics) | 0.471 📏 | 34 |
| rare meat | 0.392 | 5.6 |
| refined wheat breads | 0.392 | 126 |
| triphala | 0.392 📏 | -10 |
| Slippery Elm | 0.392 | 49.8 |
As expected, with my two masters preamble, we have disagreements. All of the mouth items came in as weak suggestions against BiomeSight suggestions (range: -460 to 465), so doing them will likely not have significant impact on the other end’s microbiome.
Back to Microbiome Suggestions, we have in decreasing priority (excluding antibiotics):
- mastic gum (prebiotic)
- lauric acid(fatty acid in coconut oil,in palm kernel oil,)
- garlic (allium sativum)
- N-Acetyl Cysteine (NAC),
- whole-grain barley
- lactobacillus kefiri (NOT KEFIR) – only available in Italy to the best of my knowledge
- rosmarinus officinalis (rosemary)
- Hesperidin (polyphenol)
- thiamine hydrochloride (vitamin B1)
- Vitamin B-12
- ibuprofen
- Arbutin (polyphenol)
- brown rice
- luteolin (flavonoid)
- cinnamon (oil. spice)
- raffinose(sugar beet)
- Nicotine (Nicotine patches?) See this story of a person recovering from using nicotine patches
- vitamin b7 biotin (supplement) (vitamin B7)
And on the to avoid
- ketogenic diet
- berberine
- stevia
- xylan (prebiotic) (as with the mouth)
- mediterranean diet
Looking at Diet Styles, only two are strong indicated, both avoids (listed above).
Food Site
The food site takes the nutrients found and assists in building a food menu plan.
| 👍 | 100 | Cystine |
| 👍 | 94.17 | Naringin |
| 👍 | 92.19 | Thiamin |
| 👍 | 90.83 | Hesperetin |
| 👍 | 88.99 | Caffeine |
| 👍 | 86.67 | Vitamin B-12 |
| 👍 | 84.71 | Vitamin C |
For the top one, a nutrient unfamiliar to most people, we see this list to choose from. We See Barley on it. It Barley is a problem, then almond, peanut or pistachio are good alternatives. For Peanuts, I actually did some posts in the ME/CFS context.
- Peanut Butter – a complex food? [2013]
- Peanuts – A recommended part of diet [2015]
- Nuts and Seeds for CFS? [2017]
Similarly, Naringin points to Grapefruit (just about the only choice) with rosmarinus officinalis (rosemary) being a vert diminished next choice.
Thiamin is an easy to find vitamin — Vitamin B1
Hesperetin is in Lime, Blond Orange, Lemon and Grapefruit (AGAIN Grapefruit!)
Questions
Q: What testing method is BristleHealth using?
A: “Shotgun microbiome test examples: Bristle (oral microbiome).” [src] Biomesight is using 16s which provides less data. Thorne uses shotgun testing and thus would be a better match.
Q: On cfsremission and/or cort johnsons blog you discussed the importance of breaking down biofilms with things like nac as well as rotating herbs, probiotics and antibiotics. Is that a layer that should be added onto the items selected by microbiomeprescription (I plan to reread those posts before starting).
A: Yes, I have posted about biofilm in the past: Combating an Infection Defense Mechanism: Biofilms [2014] and Probiotic Biofilm Breakers[2016]. It is not a simple matter “Biofilms provide survival sites for both beneficial and opportunistic pathogenic bacteria, by providing protection as above and increasing the potential of the bacteria to survive and evolve” [2013]. It impacts antibiotic resistance [2020]. In other words, we have yin-yang. If you are intending to aggressively reduce bacteria known to use biofilms, especially with antibiotics, then it is a wise choice. In most cases, I would not do it by default. For example, Akkermansia muciniphila and Lactobacillus rhamnosus GG both form biofilms [2020].
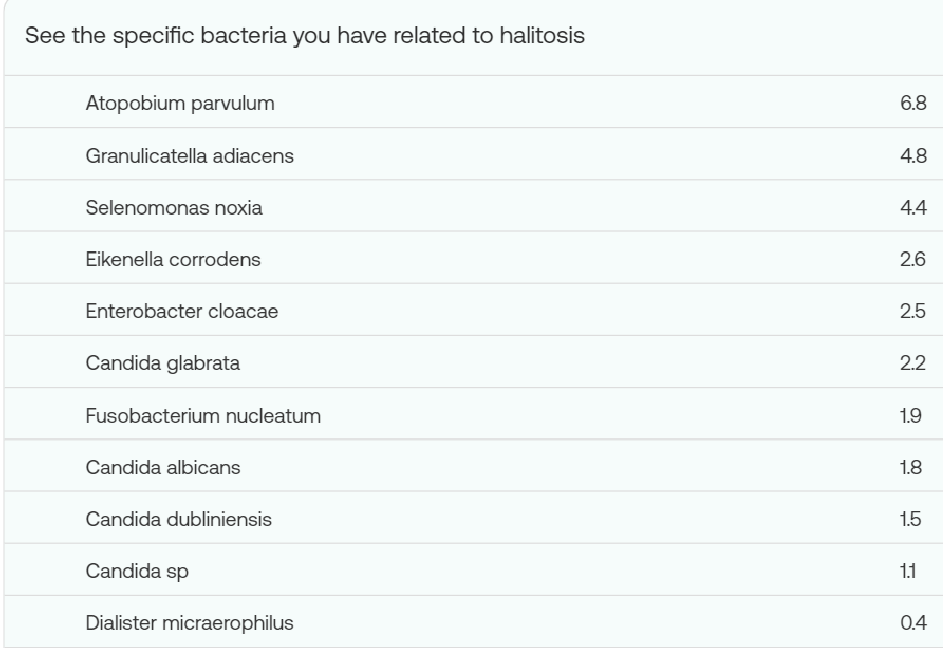
Q: What can you suggest to deal with Halitosis (Bad Breath)
A: The bacteria involved are nicely listed in your report.
We will again use All Bacteria [Genus] Reported but deem all of the above to be high (0-5), very high (5+).
In the resulting list we see many items that can be used as teas (which would likely impact the mouth bacteria): triphala, oregano (origanum vulgare, oil) . Items that can be chewed in the mouth: mastic gum .
- I personally use the hard tablets of clostridium butyricum Miyarisan which I allow to dissolve in the mouth
On the avoid: alcoholic beverages (rarely an option with ME/CFS), gluten-free diet, aspartame (sweetener).
The same approach may be done for other mouth bacteria that you wish to eliminate, you should cross check that none of the substance are strong avoid for the “other end”.
More Readings:
- Editorial: Association between oral microbiota dysbiosis and the development of systemic conditions [2023] ” there may be a bidirectional relationship between the oral microbiota and systemic diseases. “
- “The detrimental effects of oral microorganisms are not confined to the oral cavity, they can also contribute to systemic disorders, such as type 2 diabetes mellitus, cardiovascular disease, and inflammatory bowel disease (IBD) (Hajishengallis and Chavakis, 2021). “
- “In the article by Chen et al. the influence of periodontal pathogen infection on gut mycobiome was explored. The authors demonstrated the first evidence of gut fungal dysbiosis with periodontal pathogen administration. “
- The Role of the Oral Microbiota Related to Periodontal Diseases in Anxiety, Mood and Trauma- and Stress-Related Disorders [2021]
- Roles of oral microbiota and oral-gut microbial transmission in hypertension [2023]
- Oral Microbiota: A Major Player in the Diagnosis of Systemic Diseases [2021]
Bottom Line
This person’s microbiome matches the pattern usually seen with ME/CFS and Long COVID. The suggestions are also in general keeping with what has been reported to reduce symptoms. The B-Vitamins are well established. Some other citations, Brain “fog,” inflammation and obesity: key aspects of neuropsychiatric disorders improved by luteolin [2015], Modulation of antigen-induced chronic fatigue in mouse model of water immersion stress by naringin, a polyphenolic antioxidant [2009]
The oral microbiome impacting the entire flow (including SIBO) seems to be well illustrated with this data. The bacteria strains from the Bristle Health report appear to be those known to cause issues in the mouth (and most are not reported on by other tests). This implies that the ideal pair of tests to deal with systemic health issue is likely Bristle Health and Thorne.
Recent Comments